Is CBD legal in Switzerland? Overview of the Situation in 2023
In Switzerland and Europe, products containing cannabidiol (CBD) are increasingly recognized and have become part of our daily lives, mainly thanks to their positive effect on well-being. Unlike THC (tetrahydrocannabinol), Swiss CBD is not subject to narcotics law. This substance, unlike the THC molecule, does not have a psychoactive effect and does not cause a state of intoxication.
However, CBD cannot be present in any preparation or promoted
arbitrarily. Since 2017, the consumption of CBD has been legal in Switzerland, with the mandatory condition of a low THC content (less than 1% in each product). It is more commonly referred to as “light cannabis,” “legal cannabis,” or “legal CBD.”
uWeed only offers quality and legal hemp and cannabis-based products. In this article, we have prepared an overview for you on the legal framework imposed for producing and distributing legal Swiss CBD products.
Table of contents
- Is CBD cannabis legal? Is it considered a drug?
- Cannabis THC: The legislation on narcotics in Switzerland
- Smoking CBD in Switzerland: hemp-based tobacco substitute products
- Are CBD vapes and liquids legal in Switzerland?
- CBD-based products are also governed by specific standards
- Status of CBD products: Some are not approved as food
- Is there a ban on CBD oil in Switzerland?
- CBD-based therapeutic products
- CBD for Animals: What is the Legal Status?
Is CBD cannabis legal? Is it considered a drug?
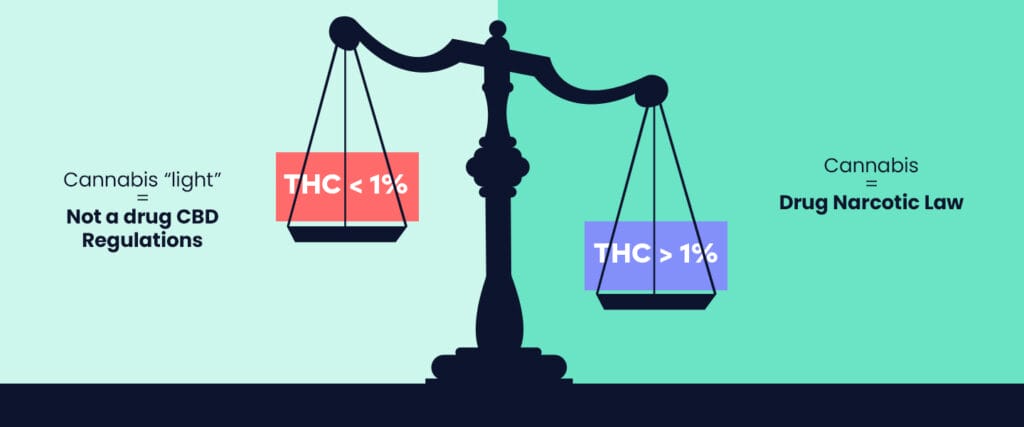
While research continues on the various effects of CBD on health, CBD cannabis has been legal in Switzerland since 2017. It has widely proven its success since then and has seen the emergence of a wide range of products favorable for well-being. Sales of CBD products are allowed by law in our country if they contain less than 1% THC and do not cause psychotropic effects. In this sense, legal Swiss CBD cannabis is not considered a drug and is not part of the list of narcotics.
However, if a control were to reveal a THC content higher than 1%, the product would be assimilated to a narcotic. Be aware that our country is more lenient than France or the rest of Europe, applying a regulation of 0.3% maximum THC within CBD consumables. This allows for better ratios and less elimination of cannabinoids or terpenes, essential for the entourage effect.
Is CBD cannabis legal in Switzerland?
Our country is more flexible than France or the rest of Europe, where the THC content in CBD consumer goods is limited to a maximum of 0.2% to 0.3%. This allows for less elimination of cannabinoids crucial for the entourage effect. Discover without delay our online sale of CBD flowers, available for quick delivery!
Cannabis THC: The legislation on narcotics in Switzerland
If you want to know more about the legality of cannabis-based products in Switzerland, you can also read our article on the legal framework of cannabis in Switzerland. This article deals only with the legality of cannabidiol (CBD) cannabis containing less than 1% THC. If the THC content is higher, Swiss law classifies cannabis as a narcotic.
Since the beginning of the narcotics law, there has been an “astonishing” clause despite all prohibitions and sanctions: Article 19b. This article stipulates that, despite the general penalization of handling products containing at least 1.0% THC, it is not an offense if someone possesses a small amount (10g was determined in 2013) for personal use. Likewise, free distribution for immediate consumption among adults is not penalized.
Furthermore, the Federal Court in Lausanne rendered a judgment on June 19, 2023 (6B_911/2021) stating that a minor amount of cannabis (up to 10 grams) for personal use cannot be confiscated for destruction by justice. This is because acquiring and possessing such a quantity for private purposes is legal.
What legislation is for low THC CBD cannabis products?
Low-THC cannabis is subject to different laws, depending on the category of finished products in which it is used. Even if CBD does not fall under the narcotics law, it cannot be distributed and promoted at will. There are different categories of products, depending on their industrial use. Thus, a CBD product may fall under the drug law or the food law and must, therefore, meet different requirements. To provide an overview and regulate the introduction of products containing cannabidiol on the market, the Federal Office of Public Health (FOPH) and Swissmedic (Swiss Institute of Therapeutic Products) have published a document allowing for a better understanding of the obligations surrounding CBD.
Smoking CBD in Switzerland: hemp-based tobacco substitute products
The Federal Court has established that hemp products containing CBD are not considered tobacco substitutes under the federal law on the taxation of tobacco. As a consumer, you, therefore, do not pay tobacco tax when you consume CBD flowers. This tax is currently about 25% in Switzerland. Apart from the fiscal aspect, CBD smoking products are, however, considered tobacco products, which is why they are 100% legal. The rules concerning when and where it is allowed to smoke CBD correspond to those of tobacco.

Are CBD flowers legal in Switzerland?
The consumption of CBD flowers, that is, smoking CBD, is legal. However, the THC content must be less than 1%, and the producer must declare the marketing to the Federal Office of Public Health. The same rule applies to CBG flowers, which focus on cannabinoids like CBG. Pre-rolled CBD joints also fall under this law.
As a reminder, any advertising statement suggesting a beneficial effect on the health of tobacco products is prohibited (Art. 17 para. 2 TabV). Other regulations applicable to tobacco also apply to CBD products. For example, protection against passive smoking must be ensured, which is why it is prohibited to smoke in enclosed or public spaces. In addition, products intended to be smoked must bear labels, such as the mention “Smoking kills.”
Buy CBD flowers according to growing method
Is CBD hash legal in Switzerland?
Hash, or resin, was long prohibited due to its many trichomes and its association with illegal cannabis, even if the CBD content was below 1%. The Federal Council had repeatedly found high concentrations of CBD and preferred to ban it. Since August 1, 2022, CBD hash is again 100% legal in Switzerland. This follows the modification of the Swiss regulation on medical cannabis.
uWeed offers you the most comprehensive selection of CBD hash and CBD pollens in Switzerland, carefully chosen to provide an exceptional aromatic experience.
Are CBD vapes and liquids legal in Switzerland?
The vaping of electronic cigarettes occupies a particular place in Switzerland. It is planned that vaporizers will fall under the tobacco products law. However, they currently fall under the food law, which poses particular problems. For example, unlike tobacco products, it is not necessary to ensure protection against passive smoking. The sale and advertising to minors are also problematic. In addition, Switzerland does not have its own rules on the requirements that electronic cigarettes must meet; they align with those of EU or EEA member states.

CBD vaping on the streets is legal in Switzerland
CBD vaping is legal in Switzerland, which means that CBD liquids and cartridges are also allowed. This follows the legislative changes in 2016, which relaxed some restrictions on cannabis-based products. The same rules apply to resins or dried flowers: they must contain less than 1% THC, and the manufacturer must indicate it. Nevertheless, CBD vaping is still regulated by Swiss legislation on everyday objects.
The marketing of cosmetics infused with CBD or legal cannabis is permitted, provided that the non-toxicity of each ingredient is proven via a detailed safety report. However, these products must not be presented as remedies.
CBD-based products are also governed by specific standards
Synthetic CBD stands out in the cosmetics industry, escaping restrictions imposed by the 1961 Narcotics Convention that prohibits the use of cannabis in cosmetics. In general, cosmetics must be limited to components extracted from leaves and seeds, excluding the use of flowers or fruits. Thus, synthetic CBD represents a legal and compliant alternative for integration into cosmetics.
You can, therefore, find many legal CBD balms and cosmetics and order them directly from our site.

Status of CBD products: Some are not approved as food
In Switzerland, the approval of CBD products as food is often hampered by legal restrictions. As we detailed earlier, the legal framework surrounding the use of CBD and hemp-based products, especially regarding smoking, is quite clear.
However, the situation becomes more complex for other products. While ingredients such as hemp seeds, oil, and hemp seed flour are generally accepted as food, this does not apply to CBD teas or oils infused with hemp extracts.
These are classified as “novel foods” (Novel Food), as it is not proven that they were consumed significantly in the EU before May 15, 1997. Moreover, it is essential not to neglect the potential side effects of CBD. This distinction underscores the need for caution in classifying and approving CBD products in the food industry.
Authorization of “Novel foods” or “Novel Food”
Cannabidiol, or “light cannabis” for some, belongs to the category of novel foods, commonly known as “Novel Food.” Any company wishing to produce, import, or distribute products containing such ingredients must prove that their consumption was significant before May 15, 1997, and that they are not considered novel foods. For example, if a company wants to market hemp oil containing CBD, it generally falls under the novel food regulation. This means that the manufacturer must obtain authorization from the FOPH or approval from the European Commission.
In 2022, various companies, united under the banner of the European Industrial Hemp Association (EIHA), joined forces to challenge the current regulation, seeking to prove the safety of CBD products to obtain food approvals. They tried to demonstrate significant consumption of CBD before 1997 to circumvent the rule on novel foods.
However, these efforts have yet to bear fruit. Most CBD oils and similar products are not recognized as food in the EU and Switzerland. For approval as food, these products must be classified as ‘Novel Food’, a recognition rarely granted. Currently, most CBD products on the market are therefore approved as cosmetics or chemical substances. Thus, they are often treated with agents like rosemary oil, particularly in the case of CBD oils.
Many CBD products contain
Consumer safety is a top priority for the Swiss Federal Council. Therefore, it regularly monitors the market release of products containing THC. The narcotics law states that the THC content in CBD foods must be below 2 mg/kg.
Since 2017, Switzerland has allowed the consumption of CBD, provided it has a low THC content (less than 1%). Commonly referred to as “light cannabis,” “legal cannabis,” or “legal CBD,” this product is gaining popularity. However, a study revealed by chemist Patrick Edder in a February 2022 RTS podcast shows concerning results: about 85% of CBD products in Switzerland were non-compliant, mainly due to incorrect categorization of cannabidiol oils as foods, an unauthorized practice as explained earlier.
hemp tea, a delicious alternative to CBD tea
For CBD enthusiasts, we offer an exquisite selection of hemp teas characterized by a low THC content. We invite you to explore our page dedicated to these varied CBD teas, combining flavor and well-being.
When a tea is purely made from hemp leaves, it is generally classified as food. However, it is expected to subject these teas to a check to determine if they constitute a ‘novel food’ or ‘Novel Food.’ It should be noted that approval becomes more complex for highly concentrated hemp teas.
Is there a ban on CBD oil in Switzerland?
At uWeed, we ensure that all our cannabidiol-based products are not only legal but also free of any substance similar to narcotics. However, it should be noted that, according to analyses by Swiss chemists, many products on the Swiss market do not meet these standards. Some of these products, approved as chemical products, should theoretically contain a denaturant, but this is often not the case, making CBD oil non-compliant.
However, this does not necessarily mean these products are harmful or contain dangerous substances. It is crucial to monitor the dosage of CBD drops carefully. At uweed.ch, we take extra measures to ensure the safety and compliance of our products. We order independent laboratory analyses at our expense to confirm their quality and compliance. Moreover, we only select legally approved products that meet our high standards, ensuring our customers’ safe and high-quality experience.
Manufacturers do not market their products as food
Manufacturers facing restrictions on selling CBD products as food adopt alternative strategies. They obtain approvals for their CBD oils as cosmetics or chemical products. This approach allows them to avoid the obstacles related to food approval, but as a result, these products are not recommended for oral consumption.
CBD-based chemical products
Without Novel Food approval, many manufacturers have introduced CBD oils, often aromatic, scented oils, as chemical products. On March 29, 2022, authorities reacted by requiring cannabidiol brands to add a denaturing substance to these products, aiming to discourage their oral consumption.$
As of September 30, 2022, all CBD oils sold as chemical products must contain such a substance, usually non-toxic but making consumption difficult, if not impossible. A typical example of such an additive is rosemary oil.
CBD oil-based products
CBD oils are largely restricted as foods in Switzerland and are not intended for oral consumption, usually being approved as skin cosmetics or chemical products. Their safety for oral consumption has not been evaluated. Some manufacturers seek to agree on CBD oils as oral hygiene products to circumvent this restriction. However, due to the risk of abuse and their high concentration, these products often do not meet Swiss standards for cosmetics. Despite this, there are products on the market with this classification.
CBD-based therapeutic products
Swiss medicine, the equivalent of the American FDA (Food and Drug Administration), regulates all drugs in Switzerland. Since research on cannabis as a medicine is still ongoing, brands are not allowed to promote the health benefits of hemp-based products without solid medical and clinical evidence. Qualified as healing promises, these health claims are only allowed with scientific validation.
CBD-based medications: Epidyolex
On June 28, 2018, the FDA marked a significant milestone by approving Epidiolex®, the first CBD-based medication in the world. This medication was introduced in Switzerland as Epidyolex® on February 10, 2021. It comes in the form of a syrup with a 10% concentration of CBD and does not contain THC. Although it is not yet reimbursed by health insurance in Switzerland, it could be in the future under certain conditions. Epidyolex® is specifically indicated for the treatment of certain forms of childhood epilepsy, such as Lennox-Gastaut syndrome and Dravet syndrome.
Manufacturing and Distribution of CBD-Containing Medications in Pharmacies
Medications containing CBD can be sold in pharmacies if they are approved by Swiss medicine. The FOPH supports this authorization. To request the approval of the distribution of medical products containing CBD, several elements are necessary:
- Complete and detailed documentation of the results of chemical and physical analyses.
- Proof of microbiological and biological tests.
- Results of conducted tests: toxicological, clinical, or pharmacological tests.
- Proof of the THC content of the product.
- Proof that the manufactured medical products are of the highest quality, effective for certain diseases, and incredibly safe to use.
The document published by Swissmedic emphasizes that for manufacturing medications containing CBD, the CBD used must be produced per the requirements of Good Manufacturing Practices (GMP).
For patients, certain documents are also required to be able to consume medications containing CBD:
- A medical prescription.
- An accompanying consultation from the prescribing doctor and the transmission of collected data to the FOPH.
Note that in all cases, the costs of medications containing THC or CBD are not reimbursed. Monthly costs can quickly reach four figures depending on the daily amount consumed.
CBD for Animals: What is the Legal Status?
Hemp-based animal foods, such as CBD oil for cats, can be authorized, like CBD teas or infusions for humans. However, it is essential to make a clear distinction:
- It is recommended to consult a veterinarian before introducing these products into your animals’ diet.
- If the THC content is below 1%, hemp-based foods are generally safe for dogs and cats. However, cats have a low tolerance to terpenes, hence the need for specific CBD products.
- CBD products are prohibited for lactating animals producing milk intended for human consumption (such as cows or goats).
- The regulation on animal feed prohibits the use of hemp in horses, which are still considered farm animals despite their status as domestic animals.
- In addition to oral consumption, topical use of CBD can benefit certain conditions, such as inflammation in domestic animals.
Although CBD for domestic animals is allowed under certain conditions, it is essential to respect specific restrictions and always consult a health professional.





